This is an old revision of the document!
Table of Contents
Compare different preparation protocols
Analysis description
In this use case, we will work with two biological replicates (named “Replicate1” and “Replicate2”).
Each sample is aliquoted in three parts: each of them is prepared using a different separation protocol (“Ultrafiltration”, “Proteominer” and “TCA Precipitation”).
Each aliquote is then deposed onto a SDS-Page gel.
When separating is done, each interesting gel band is extracted and analysed using HPLC-MSMS+Mascot+IRMa. Each band will result in one identification file (“F093496.dat”). So, the analysis of a given replicate will generate many identification files.
...Project ABC
...Replicate1
.Ultrafiltration
.F093496.dat
.F093497.dat
...
.Proteominer
...
.TCA Precipitation
...
...Replicate2
.Ultrafiltration
...
.Proteominer
...
.TCA Precipitation
...
Project creation in hEIDI
Please follow the steps in the getting start to setup the MSI db connection (Mass Spectrometer Identification database), create an hEIDI project and open a working session on the MSI db.
Check the filter parameters used in identification results
 verifier les paramètres de filtres des identification (how to view ident properties + .. view filter )
verifier les paramètres de filtres des identification (how to view ident properties + .. view filter ) 
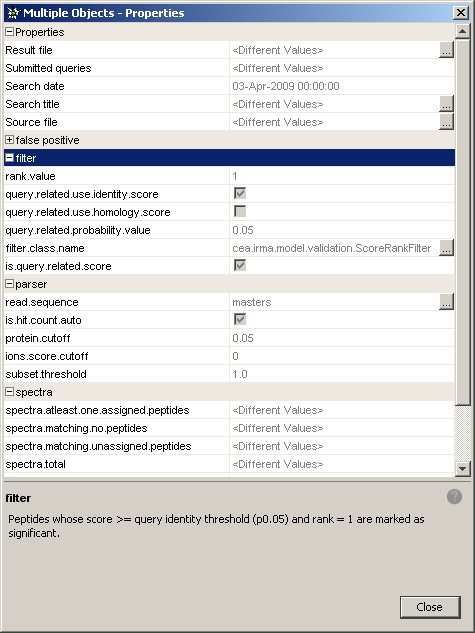 First of all, you may want to check if your identification results have been filtered in the same way. To do this:
First of all, you may want to check if your identification results have been filtered in the same way. To do this:
- Go the the
Identificationstab (you can retreive it from the main menuWindow > Identifications). - Select multiple identifications using the
Shiftkey (orCtrlkey for a disjoinct selection). - Right-click to display the context sensitive menu and select
properties. - A window will show up displaying all the properties for these identifications. There are different sets of properties (general properties, false pasitive, filter, etc.) and each of them can be collapsed/expanded. If
<different values>appears for a given property, it means that this property is different for the group of selected identifications. If the property is the same for all the selected identifications, the property value appears.
- Scroll down to the 'filter' category and check the filter properties you are interested in.
Structure your experiment as a context tree
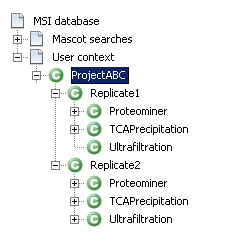 Now you need to build a context hierarchy and dispatch your identification results to map the project structure described in the first section.
Now you need to build a context hierarchy and dispatch your identification results to map the project structure described in the first section.
See the following links to know what is a context and how to create a context hierarchy.
Execute protein grouping for every contexts
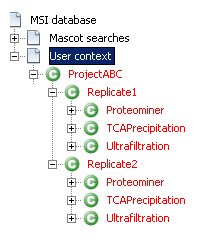 Protein grouping is the next step. Execute the protein grouping algorithm for all the User contexts in a specific order.
Protein grouping is the next step. Execute the protein grouping algorithm for all the User contexts in a specific order.
- Start with the contexts that directly group the identification results: “Ultrafiltration”, “Proteominer” and “TCA Precipitation”. Do that for the two replicates.
- Then continue with the contexts “Replicate1” and “Replicate2”.
- And finally, end with the root context named “ProjectABC”
If everything has worked correctly, all the context names are now in red.
Browse the context tree and export it
Check reproducibility of results
You may want to compare results from the same preparation protocol in the two replicates.
Compare two contexts with each other and export comparison results
 a deplacer
a deplacer 
How to compare contexts each other.
See the following link to understand what is context comparison.
Find the optimal preparation protocol
You may want to compare protocols each other for a given replicate to find the “best” one.
Compare a parent context with their child contexts and export comparison results
 a deplacer
a deplacer 
- Show covering diagram
- Export results
See the following link to understand what is context comparison.

